Tip Cell biology and Vessel Guidance
|
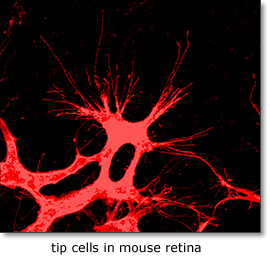
Remodeling of developing embryonic and adult vascular networks requires navigation of blood vessels through tissue and their precise projection and connection to target cells and organs. The cellular and molecular mechanisms accounting for this type of directed angiogenesis, shows striking similarities with axon guidance in the developing nervous system. Sprouting blood vessels are spearheaded by specialized tip cells, which navigate vessels using their filopodia to sense gradients of guidance cues . While VEGF-A acts as an attractant cue, tip cells also express neural guidance receptors including Unc5B and Plexins that mediate repulsive guidance events in response to netrins and class-3 semaphorins. Periperal nerve derived VEGF may further fine-tune arterial branching morphogenesis. VEGF receptors including Flk1 (Kdr/VEGF receptor-2), Flt4 (VEGF receptor-3), Flt1 (VEGF receptor-1) and Notch-delta like 4/Jagged1 (Dll4/Jag1) signaling pathways play key roles in tip cell formation. Within the angiogenic sprout, endothelial cells compete via bilateral Dll4-Notch signaling involving a VEGF-Notch feedback loop for tip cell specification. Growing sprouts also secrete molecules that regulate tip cell behavior including the neuropeptide apelin and soluble Flt1, and extrinsic neuronal Dll1 may further fine-tune tip cell selection. At present the full repertoire of genes controlling endothelial tip-stalk cell differentiation and cell movements within the sprout is unknown. |
 |
Research focus 1: Flt1 and vascular development
We recently showed that Flt1 (VEGF receptor-1) is expressed in both vessels and nerves, and Flt1 regulates angiogenic remodeling, and neuronal differentiation events (Krueger et al, Development 2011). In loss and gain of function studies in zebrafish embryos we find that Flt1 modulates tip cell differentiation and vessel branching by acting on Notch receptor expression in angiogenic sprouts.
We furthermore find that the developing nervous systems may contribute to the distribution of (soluble) Flt1, and loss of Flt1 associated with neuronal differentiation defects. Here we aim at elucidating the molecular mechanism regulating Flt1 splicing, Flt1 signaling in vessels, and the role of soluble Flt1 at the neuro-vascular interface in the context of vessel guidance.
Research focus 2: Flt1 and Angioneurins
Formation of vascular and neuronal networks is dependent on common molecular cues and utilizes the same principle of directed guidance. Furthermore, the patterning of blood vessels and nerves is interdependent. Factors originally reported to act as guidance factors in the vascular system (such as vascular endothelial growth factor (VEGF)), are also implicated to affect neurogenesis. Developing nerves secrete factors like VEGF relevant for arterial differentiation and vessel guidance, and growing arteries secrete nerve growth factors determining their innervation and functionality. We recently found that VEGF-receptor-1 - Flt1 - has dual neurovascular properties in zebrafish embryos. Such molecules are called Angioneurins Here, we propose to systematically analyze the role of Flt1, and Flt1 ligands in neuronal differentiation, axon guidance, and cross-talk with vascular patterning using the zebrafish model system.
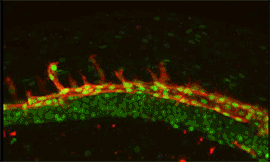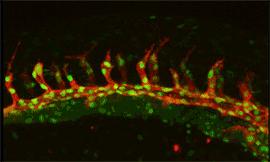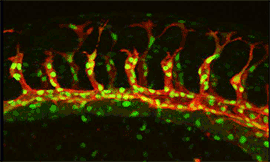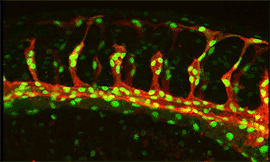
Research focus 3: Post-transcriptional regulation of Flt1
We showed that Flt1 is spliced in two forms: membrane bound Flt1 (mFlt1) and soluble Flt1 (sFlt1). These forms are differentially expressed in arteries/veins and certain neuronal compartments. Loss and gain of function approaches suggest differences in their impact on vascular and neuronal growth. We would like to identify the factors that contribute to tissue Flt1 splicing events, and their impact on vessel / neuronal patterning. We furthermore obtained evidence that Flt1 can be regulated post-transcriptionally by microRNAs, epigenetic modifiers and splicing factors.
Using state of the art techniques we aim to identify and functionally characterize the post-transcriptional regulators of Flt1 using bio-informatics, sequencing analysis, and RBP-RNA analyses substantiated by loss/gain of function studies in transgenic zebrafish.
Publications
- Ziegler, T., R. Hinkel, A. Stohr, T. Eschenhagen, K.-L. Laugwitz, F. le Noble, R. David, A. Hansen, and C. Kupatt (2017) Thymosin 4 Improves Differentiation and Vascularization of EHTs. Stem cells international, v. 2017, p. 6848271.
- Ziegler, T., M. Kraus, W. Husada, F. Gesenhues, Q. Jiang, O. Pinkenburg, T. Trenkwalder, K.-L. Laugwitz, F. le Noble, C. Weber, C. Kupatt, and R. Hinkel (2017) Steerable Induction of the Thymosin beta4/MRTF-A Pathway via AAV-Based Overexpression Induces Therapeutic Neovascularization. Human gene therapy
- Wild R, Klems A, Takamiya M, Hayashi Y, Strähle U, Ando K, Mochizuki N, van Impel A, Schulte-Merker S, Krueger J, Preau L, le Noble F (2017) Neuronal sFlt1 and Vegfaa determine venous sprouting and spinal cord vascularization. Nature Communications. 8:13991. DOI: 10.1038/ncomms13991
- Lange C, Turrero Garcia M, Decimo I, Bifari F, Eelen G, Quaegebeur A, Boon R, Zhao H, Boeckx B, Chang J, Wu C, Le Noble F, Lambrechts D, Dewerchin M, Kuo CJ, Huttner WB, Carmeliet P. (2016) Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J. 2016 May 2;35(9):924-41. doi: 10.15252/embj.201592372. Epub 2016 Feb 8. PMID: 26856890
- Hinkel R., Trenkwalder T., Petersen, B., Husada W., Gesenhues F., Lee S., Hannappel E., Bock-Marquette I., Theisen D., Leitner L., Boekstegers P., Cierniewski C., Müller O., Le Noble F., Adams R., Weinl C., Nordheim A., Reichart B., Weber C., Olson E., Posern G., Deindl E., Niemann H., and Kupatt C. (2014). MRTF-A controls vessels growth and maturation by increasing the expression of CCN1 and CCN2. Nature Communications. 5:3970. DOI:10.1038/ncomms4970
- Le Noble F., and Le Noble J. (2014). Bone biology: Vessels of rejuvenation. Nature 507, 313-4.
- Cristofaro B., Shi Y., Faria M., Suchting S., Leroyer A., Trindade A., Duarte A., Zovein A., Iruela-Arispe M., Nih L., Kubis N., Henrion D., Loufrani L., Todiras M., Schleifenbaum J., Gollasch M., Zhuang Z., Simons M., Eichmann A., and Le Noble F. (2013) Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development 140, 1720-9
- Jiang Q, Lagos-Quintana M, Liu D, Shi Y, Helker C, Herzog W, le Noble F. (2013) miR-30a regulates endothelial tip cell formation and arteriolar branching. Hypertension 62, 592-8
- Dimova I., Hlushchuk R., Makanya A., Styp-Rekowska B., Ceausu A., Flueckiger S., Lang S., Semela D., Le Noble F., Chatterjee S., and Djonov V. (2013). Inhibition of Notch signaling induces extensive intussusceptive neo-angiogenesis by recruitment of mononuclear cells. Angiogenesis 16, 921-37
- Napp, L.C., Augustynik, M., Paesler, F., Krishnasamy, K., Woiterski, J., Limbourg, A., Bauersachs, J., Drexler, H., Le Noble, F., Limbourg, F.P. (2012). Extrinsic Notch ligand Delta-like 1 regulates tip cell selection and vascular branching morphogenesis. Circulation Research 110, 530-5
- Krueger, J., Liu, D., Scholz, K., Zimmer, A., Shi, Y., Klein, C., Siekmann, A., Schulte-Merker, S., Cudmore, M., Ahmed, A., and Le Noble, F. (2011). Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development 138, 2111-2120
- Hillmeister, P.*, Gatzke, N.*, Dulsner, A., Bader, M., Schadock, I., Hoefer, I., Hamann, I., Infante-Duarte, C., Jung, G., Troidl, K., Urban, D., Stawowy, P., Frentsch, M., Li, M., Nagorka, S., Wang, H., Shi, Y., Le Noble, F*., and Buschmann, I*. (2011). Arteriogenesis Is Modulated By Bradykinin Receptor Signaling. Circulation Research 109, 524-533. *equal contribution
- Klein, C., Mikutta, J., Krueger, J., Scholz, K., Brinkmann, J., Liu, D., Veerkamp, J., Siegel, D., Abdelilah-Seyfried, S., and Le Noble, F. (2011) Neuron navigator 3a regulates liver organogenesis during zebrafish embryogenesis. Development 138, 1935-1945
- Liu, D., Krueger, J., and Le Noble, F. (2011) The role of blood flow and microRNAs in blood vessel development. International Journal of Developmental Biology 55, 419-429
- Buschmann, I., Pries, A., Styp-Rekowska, B., Hillmeister, P., Loufrani, L., Henrion, D., Shi, Y., Duelsner, A., Hoefer, I., Gatzke, N., Wang, H., Lehmann, K., Ulm, L., Ritter, Z., Hauff, P., Hlushchuk, R., Djonov, V., van Veen, T., and Le Noble, F. (2010). Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development 137, 2187-2196
- Pries, A., Höpfner, M., le Noble, F., Dewhirst, M. W., Secomb, T. W. (2010) The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer. 2010 Aug; 10(8): 587–59
