Wnt in Xenopus
Research
Signal transduction triggered by proteins of the Wnt family is a common theme in development (http://web.stanford.edu/group/nusselab/cgi-bin/wnt/). Wnt signaling pathways trigger embryonic axis formation, patterning of the central nervous system and cell movements during gastrulation. Signal transduction is induced by binding of a Wnt ligand to its Fz receptor, the recruitment of a co-receptor and the formation of a signalosome. The composition of this signalosome defines which branch of the signal network gets activated.
|
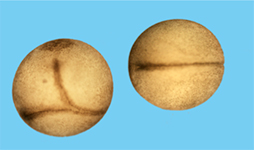
Activation of the canonical (or Wnt/β-Catenin) pathway results in the accumulation of the transcriptional co-activator β-Catenin. This β-Catenin interacts with transcription factors of the Tcf/Lef family to control the expression of Wnt target genes. Vertebrates express four different Tcf/Lef family members, each of them with its one, non-redundant function in early development. We aim to identify molecular mechanisms underlying the differences of the four Tcf/Lef transcription factors (König et al., 2010, Klingel et al., 2012). |
 |
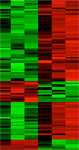 |
The coordinated activation of at least three different branches of the Wnt signaling network regulates convergent extension movements during Xenopus gastrulation. In this process, Wnt11 (Wnt/PCP pathway) triggers the polarization of the cells, a necessary step for their migration towards the midline, Wnt5a (Wnt/ROR pathway) is important for the extension. To elucidate the impact of the different branches of the Wnt signaling network we performed transcriptome analyses of Xenopus morphants where distinct branches of the network were knocked down. |
| Currently we analyze how selected target genes specifically regulated by distinct branches of the Wnt signaling network regulate convergent extension movements. | 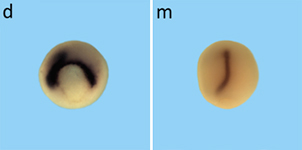 |
| Of particular interest is, whether these novel target genes influence clustering of ROR2/Wnt5a in the membrane of dorsal marginal zone explants. Together with U. Nienhaus from the Institute of Applied Physics (KIT) we use advanced fluorescence microscopy techniques to determine steady state levels and binding constants of non-canonical Wnt ligands binding to their receptors/co-receptors (Hedde et al., 2013, Wallkamm et al., 2014). |  |
| To analyze the impact of the different Wnt branches on angiogenesis and lymphangiogenesis in Xenopus we systematically knock-down and overexpress various Wnt-pathway components and follow up the expression patterns of selected vessel-specific marker genes. Selected components shall be analyzed in transgenic Xenopus embryos expressing GFP under the control of the fli promoter. | 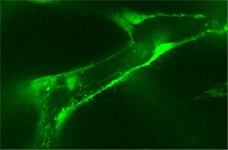 |
Publications
2017-2012
- Wallkamm, V., Rahm, K., Schmoll, J., Kaufmann, L.T., Brinkmann, E., Schunk, J., Kraft, B., Wedlich, D., Gradl, D. (2016). Regulation of distinct branches of the non-canonical Wnt-signaling network in Xenopus dorsal marginal zone explants. BMC Biol. 14:55
- Brinkmann, E.M., Mattes, B., Kumar, R., Hagemann, A.I., Gradl, D., Scholpp,S., Steinbeisser, H., Kaufmann, L.T., Ozbek, S. (2016). Secreted frizzled-related protein 2 (sFRP2) redirects non-canonical Wnt signaling from Fz7 to Ror2 during vertebrate gastrulation. J Biol Chem. 291(26):13730-42
- Mittag, S., Valenta, T., Weiske, J., Bloch, L., Klingel, S., Gradl, D., Wetzel, F., Chen, Iver Y. Petersen, I., Basler, K., and Huber, O. (2016). LA novel role for the tumour suppressor Nitrilase1 modulating the Wnt/β-catenin signalling pathway. CellDiscovery 2, Article number: 15039.
- Loregger A, Grandl M, Mejías-Luque R, Allgäuer M, Degenhart K, Haselmann V, Oikonomou C, Hatzis P, Janssen KP, Nitsche U, Gradl D, van den Broek O, Destree O, Ulm K, Neumaier M, Kalali B, Jung A, Varela I, Schmid RM, Rad R, Busch DH, Gerhard M. (2015). The E3 ligase RNF43 inhibits Wnt signaling downstream of mutated β-catenin by sequestering TCF4 to the nuclear membrane. Sci Signal. 8(393):ra90.
- Deichmann C, Link M, Seyfang M, Knotz V, Gradl D, Wedlich D.(2015). Neural crest specification by prohibitin1 depends on transcriptional regulation of prl3 and vangl1. Genesis. 53(10):627-639.
- Schmitt, M., Metzger, M., Gradl, D., Davidson, G., Orian-Rousseau, V. (2015). CD44 regulates the Wnt pathway at the level of LRP6. Cell Death Differ, 22(4):677-89.
- Wallkamm, V., Dörlich, R., Rahm, K., Klessing, T., Nienhaus, G.U., Wedlich, D., Gradl, D. (2014). Live imaging of Xwnt5A-ROR2 complexes. PlosOne, 9(10):e109428.
- Kumar, S., Žigman, M., Patel, T.R., Trageser, B., Gross, J. C., Rahm, K., Boutros, M., Gradl, D., Steinbeisser, H., Holstein, T., Stetefeld, J. and Özbek, S. (2014). Molecular dissection of Wnt3a-Frizzled8 interaction reveals essential and modulatory determinants of wnt signaling activity. BMC Biol. 12:44.
- Tumova, L., Pombinho, A.R., Vojtechova, M., Stancikova, J., Gradl, D., Krausova, M., Sloncova, E., Horazna, M., Kriz., V., Machonova, O., Jindrich, J., Zdrahal, Z., Bartunek, P., Korinek, V. (2014). Monensin Inhibits Canonical Wnt Signaling in Human Colorectal Cancer Cells and Suppresses Tumor Growth in Multiple Intestinal Neoplasia Mice. Mol. Cancer Ther. 3(4):812-822.
- Schneider, M., Huang, C., Becker, S.F., Gradl, D., Wedlich, D.(2014).The protocadherin PAPC is expressed in the CNC and can compensate for the loss of PCNS. Genesis 52(2):120-126.
- Hedde, P.N., Dörlich, R.M., Blomley, R., Gradl, D., Oppong, E., Cato, A.C.B. and Nienhaus, G.U. (2013). Stimulated Emission Depletion-Based Raster Image Correlation Spectroscopy (STED-RICS) – A Versatile Method for Studying Biomolecular Dynamics in Live Cells. Nature Comm. Jun 27;4:2093.
- Crosta, N., Müller, S., Gradl, D., Masters, K.-S. and Bräse, S. (2013). Rediscovering the Double Friedel-Crafts Acylation: An Expedient Entry to Phenanthrene-9,10-diones. Synlett 24(8): 951-954. DOI: 10.1055/s-0032-1318481.
- Halbedl, S., Kratzer, M.-C. Rahm, K., Crosta, N. Masters, K.-C. Zippert, J., Bräse, S. and Gradl, D. (2013). Synthesis of novel inhibitors blocking Wnt signaling downstream of b-Catenin. FEBS Lett. 587(5), 522-527.
- Horn, E.R., El-Yamany, N.A. and Gradl, D. (2013). The vestibuloocular reflex of tadpoles (Xenopus laevis) after knock-down of the isthmus related transcription factor XTcf-4.J Exp Biol.216(Pt 4), 733-741.
- Kim, C., Kreppenhofer, K., Kashef, J., Gradl, D., Herrmann, D., Schneider, M., Ahrens, R., Guber, A. and Wedlich, D. (2012). Diffusion- and convection-based activation of Wnt/β-catenin signaling in a gradient generating microfluidic chip. Lab Chip, 12(24):5186-5194.
- Klingel, S., Morath, I., Strietz, J., Menzel, K., Holstein, T.W., Gradl, D. (2012). Subfunctionalization and neofunctionalization of vertebrate Lef/Tcf transcription factors. Dev Biol. 368(1), 44-53.
- Holzer, T., Liffers, K., Rahm, K., Trageser, B., Ozbek, S., Gradl, D. (2012). Live imaging of active fluorophore labelled Wnt proteins. FEBS Lett. 586(11), 1638-1644.
- Waaler, J., Machon, O., Tumova, L., Dinh, H., Korinek, V., Wilson, S.R., Paulsen, J.E., Pedersen, N.M., Eide, T.J., Machonova, O., Gradl, D., Voronkov, A., von Kries, J.P., Krauss, S. (2012). A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 72(11), 2822-2832.
2011-2004
- Doubravska , L., Krausova, M., Gradl, D. Vojtechova, M., Tumova, A., Lukas, J., Valenta, T., Pospichalova, V., Fafilek, B., Plachy, J., Sebesta, O. and Korinek, V. (2011) Fatty acid modification of Wnt1 and Wnt3a at serine is prerequisite for lipidation at cysteine and is essential for Wnt signalling. Cell Signal. 23(5):837-48.
- Waaler, J., Machon, O., von Kries, J.P., Wilson, S.R., Lundenes, E., Wedlich, D., Gradl, D., Paulsen, J.E., Machonova, O., Dembinski, J.L., Dinah, H. and Krauss S. (2011). Novel Synthetic Antagonists of Canonical Wnt Signaling Inhibit Colorectal Cancer Cell Growth. Cancer Res. 71(1), 197-205.
- Koenig, S.F., Brentle, S., Hamdi, K., Fichtner, D., Wedlich, D. and Gradl, D. (2010). En2. Pax2/5 and Tcf-4 transcription factors cooperate in patterning the Xenopus brain. Dev. Biol. 340, 318-328.
- Herrmann M, Neuberth N, Wissler J, Pérez J, Gradl D, Naber A. (2009). Near-field optical study of protein transport kinetics at a single nuclear pore. Nano Lett. 9, 3330-3336.
- van Venrooy S., Fichtner D., Kunz M., Wedlich D., Gradl D. (2008). Cold-inducible RNA binding protein (CIRP), a novel XTcf-3 specific target gene regulates neural development in Xenopus. BMC Dev Biol. 8:77
- Koenig, S.F., Lattanzio, R. Mansperger, K, Rupp, R.A.W., Wedlich, D. and Gradl, D. (2008). Autoregulation of XTcf-4 depends on a Lef/Tcf site on the XTcf-4 promoter. Genesis, 46, 81-86.
- Bryja, V., Gradl, D., Schambony, A., Arenas. E., and Schulte, G. (2007). β-arrestin is a necessary component of wnt/β-catenin signalling in vitro and in vivo. Proc.Natl.Acad.Sci USA 104, 6690-6695.
- Pukrop, T., Klemm, F., Hagemann, T., Gradl, D., Schulz, M., Siemes, S., Trümper, L., and Binder, C. (2006). Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc.Natl.Acad.Sci USA 103, 5454-5459.
- Ghogomu, S., van Venrooy, S., Ritthaler, M., Wedlich, D. and Gradl D. (2006). Hic-5 a novel regulator of Lef/Tcf activity modulates the crosstalk between Wnt-signalling and steroid receptor activity. J. Biol. Chem. 281, 1755-1764.
- Winkler, C., Eggert, C., Gradl, D., Meister, G., Giegerich, M., Wedlich, D., Laggerbauer, B. and Fischer, U. (2005). Reduced production of spliceosomal U snRNPs as a major cause of cell death in SMN deficient cells. Genes Dev. 19, 2320-2330.
- Etard, C., Gradl, D., Kunz, M., Eilers, M. and Wedlich, D. (2005). Pontin and Reptin regulate cell proliferation in early Xenopus embryos in collaboration with c-Myc and Miz-1. Mech. Develop. 122, 545-556.
- Schambony, A., Kunz M. and Gradl, D. (2004). Cross-regulation of Wnt-signalling and cell adhesion. Differentiation. 72, 307-318.
- Kunz, M., Herrmann, M., Wedlich, D. and Gradl, D. (2004). Autoregulation of canonical Wnt signaling controls midbrain development. Dev. Biol. 273, 390-341.
< 2004
- Gradl, D., König, A. and Wedlich, D. (2002). Secondary axis formation by LEF/TCF transcription factors depends on the presence of an activating element which is flanked by two repressing motifs. J. Biol. Chem. 277, 14159-14171.
- Gradl, D. and Wedlich, D. (2002). The nuclear endpoint of the oncogenic Wnt/ß-catenin pathway: How to achieve specificity. Signpost reviews, Recent Research Developments in Molecular & Cellular Biology, S.G. Pandalai, Editor.
- Pukrop, T., Gradl, D., Heningfield, K., Knöchel, W., Wedlich, D. and Kühl, M. (2001). Identification of two regulatory elements within the high mobility group box transcription factor XTCF-4. J. Biol. Chem. 276, 8968-8978.
- König, A., Gradl, D., Kühl, M. and Wedlich, D. (2000). The HMG-box transcription factor XTcf-4 demarcates the forebrain-midbrain boundary. Mech. Develop. 93, 211-214.
- Gradl, D., Kühl, M. and Wedlich, D. (1999). The Wnt/wg signal transducer ß-catenin controls fibronectin expression. Mol. Cell Biol. 19, 5576-5587.
- Gradl, D., Kühl, M. and Wedlich, D. (1999). Keeping a close eye on Wnt-1/wg signaling in Xenopus. Mech. Develop. 86, 3-15.
